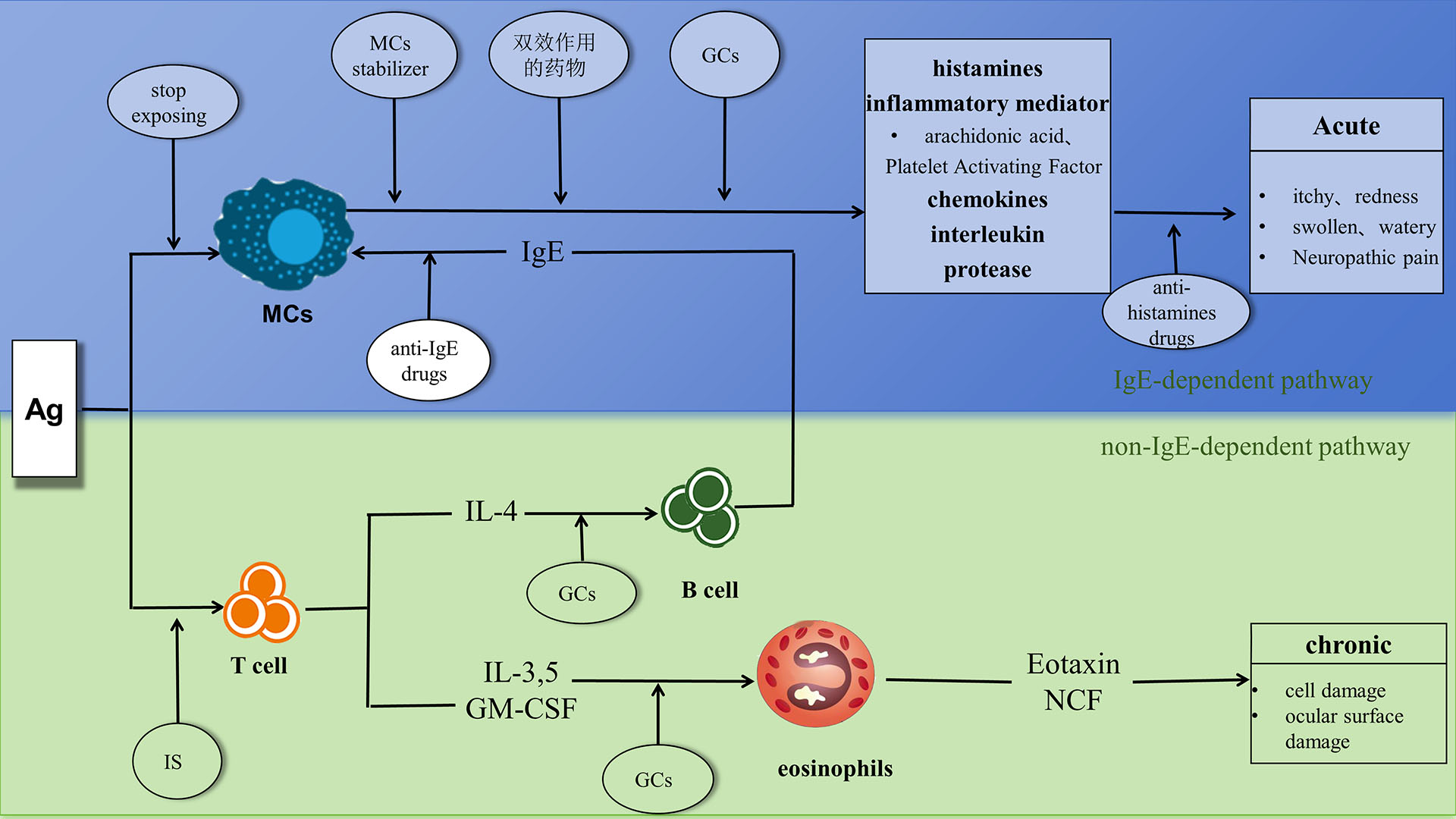
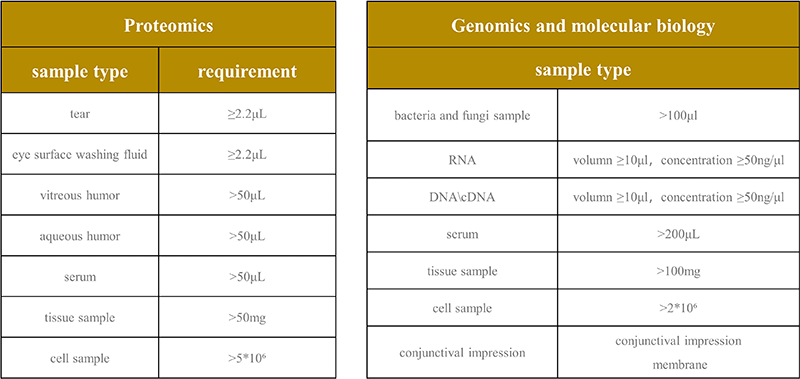
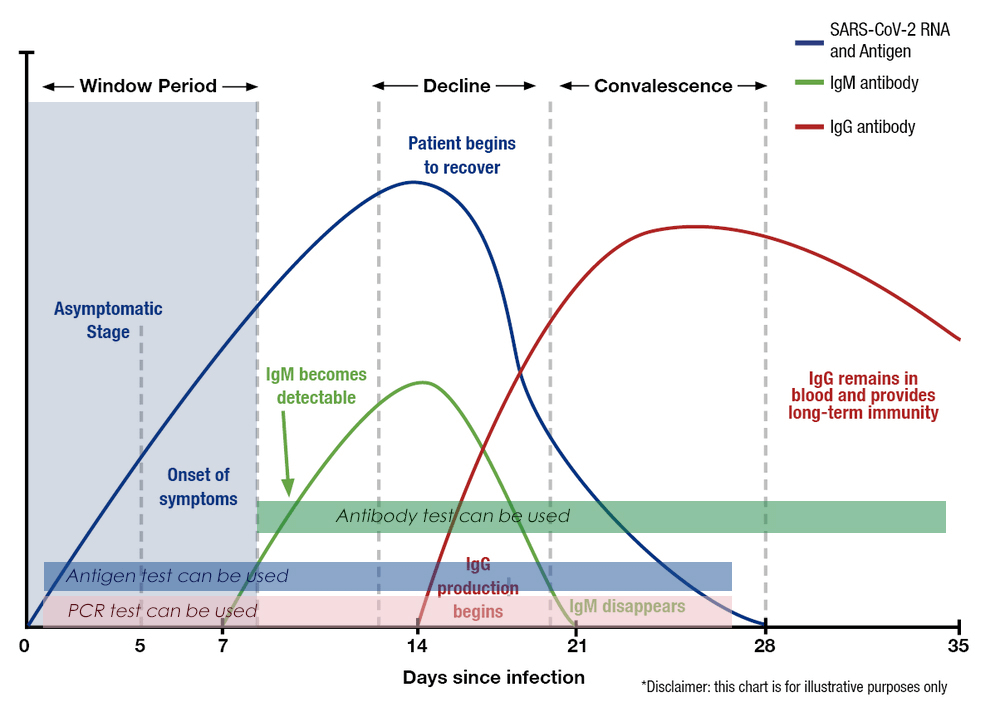
Time-consuming and complex:
The test procedure and sample processing required for most PCR tests are
labor-intensive and require specialized equipment and well-trained
personnel.
Limited flexibility:
PCR testing is primarily performed in a laboratory setting, meaning samples
must be transported to adequate facilities and the time-to-results is
delayed.
Expensive equipment:
PCR equipment can be costly, which means it is less accessible, especially for
communities that are already limited in resources and infrastructure.
Nucleic acid is extremely sensitive and unstable:
For most PCR tests, accurate results are rely on the nucleic acid remaining
intact. Transportation and handling of samples increases the risk of sample
degradation, which leads to inaccurate results.
Compared with PCR testing, antigen tests can offer a better
pathway to implementing widespread testing by:
Removing time-consuming and labor-intensive sample processing during the test
procedure, lowering the chances of user error to get faster, more reliable
results;
Eliminating the need for specialized equipment or trained personnel, while
reducing the labor costs, making testing more accessible for all;
Relieving the pressure off of hospitals and care centers, which are already
working overcapacity, by moving testing to the site of sample collection, or
other locations.
Simple, equipment-free antigen tests like CoVisAg offer
fast, portable testing and deliver results within 30 minutes of sample collection.
CoVisAg requires no additional equipment, meaning that tests can be performed
directly at the point of collection or at the patient’s side. It also reduces
the time and labor costs for testing as well as the delays for getting results
back, making it the most suitable test method for implementing widespread
testing.