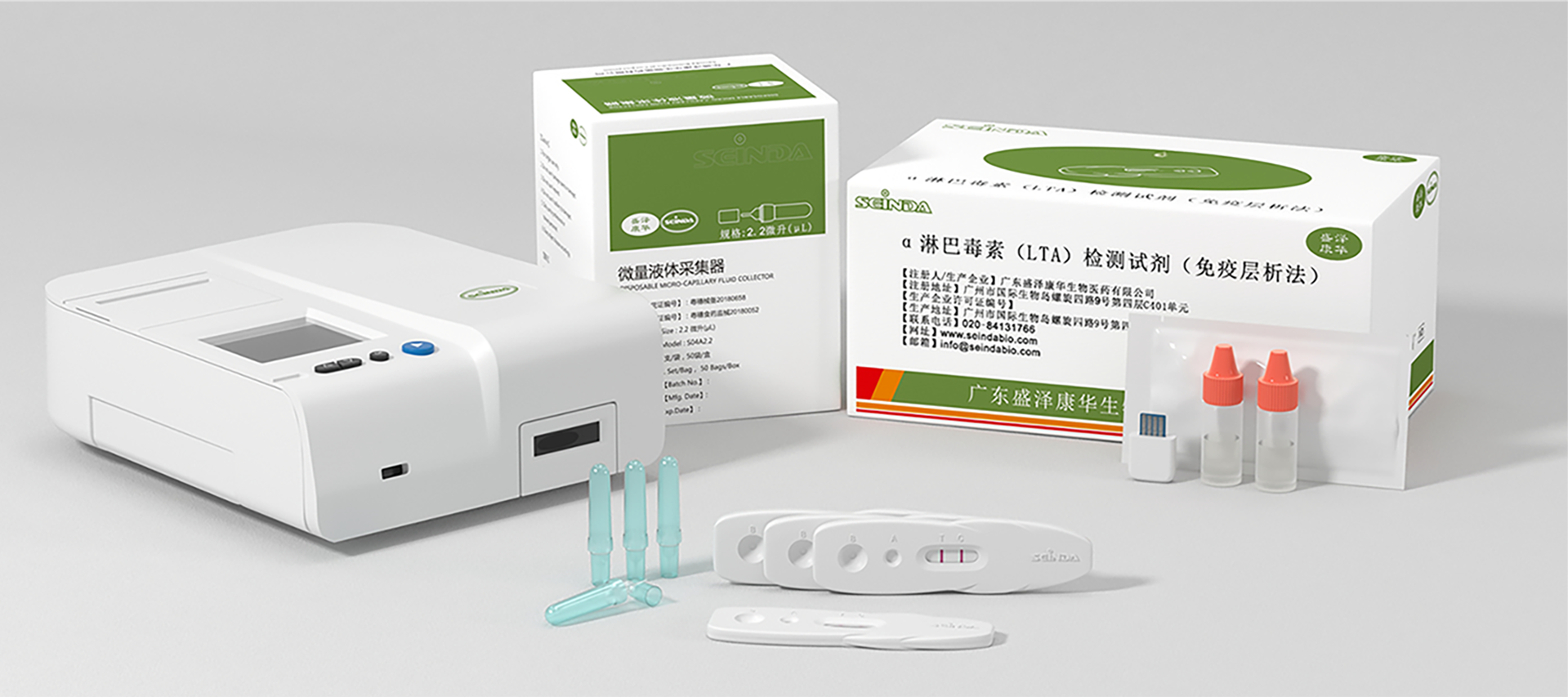
i-ImmunDx™ Platform and POCT Products
Seinda's proprietary i-ImmunDx™ platform is specifically designed for ophthalmic
point-of-care
applications. The platform uses colored nanoparticles as labelling agents coupled with
double-sandwiched
antibodies in specially designed lateral flow cassettes. Needing only 1-2 microliters of
samples, the i-ImmunDx™ platform provides
quantitative results for the detection of biomarkers, at amounts less than 1 picogram,
within 10
to 15 minutes.
The platform is the basis for the development of Seinda's series of point-of-care testing (POCT) products, which enable physicians to detect etiology of ocular disorders and procure objective, standardizable, quantitative measurements.
The platform is the basis for the development of Seinda's series of point-of-care testing (POCT) products, which enable physicians to detect etiology of ocular disorders and procure objective, standardizable, quantitative measurements.



Micro-Capillary
Fluid Collector
Fluid Collector
POC Test Kits
i-ImmunDx™ Analyzer
Micro-Capillary Fluid Collector
The Disposable Micro-Capillary Fluid Collector (DMFC) is an innovative design for
easy
and precise collection of small fluid quantities.

POC Test Kits
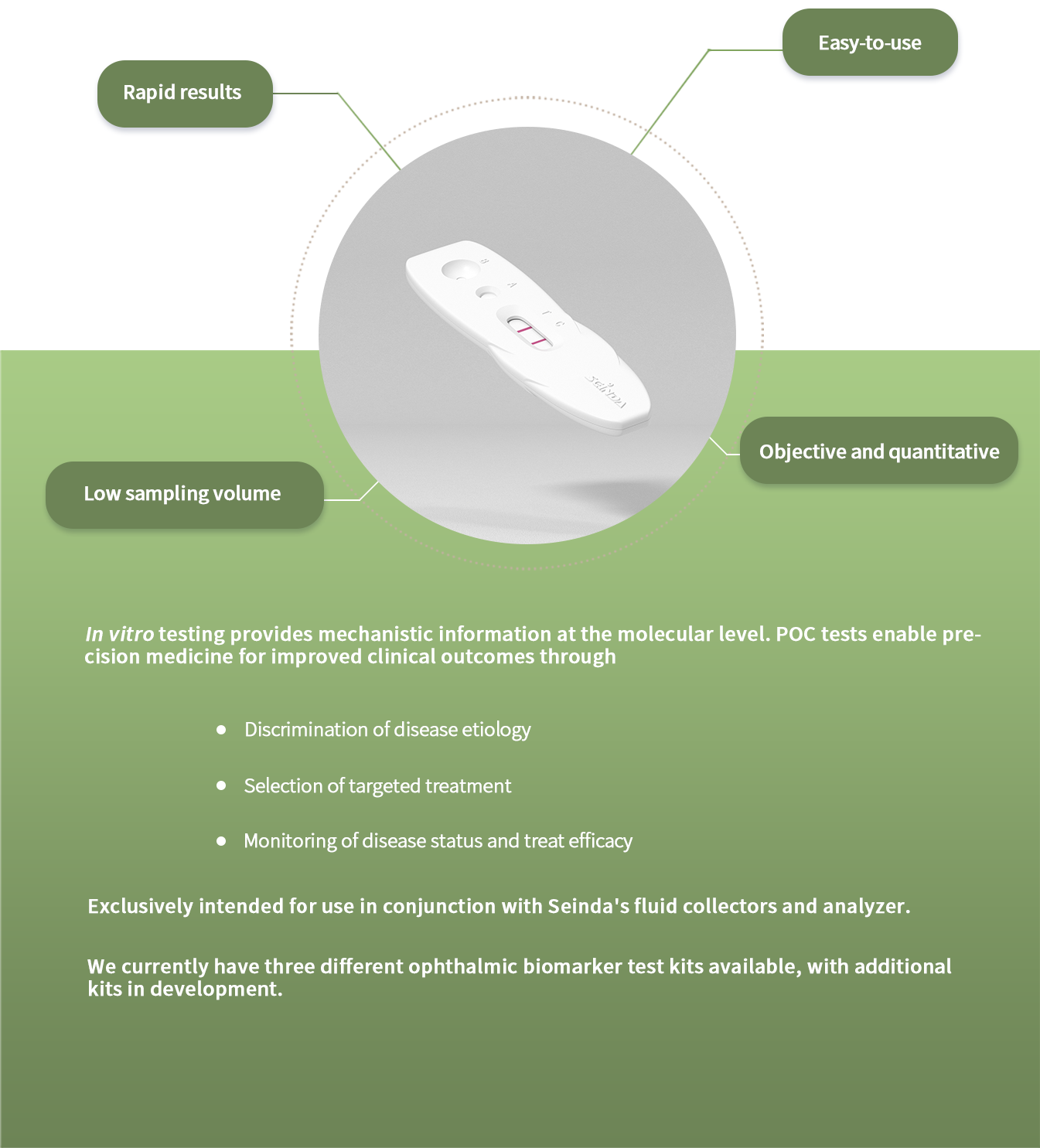
Our line of POC Test Kits facilitate in vitro
diagnosis
of
ocular disorders by measuring the quantity of specific biomarkers in tear
samples.
The
test kits are simple to perform and require only 1.0 – 2.2μL of tear fluid.
Quantitative measurements with good
precision
Rapid (
<15min) results at point-of-care
High sensitivity and specificity
LTαPOC
IgE POC
MMP-9 POC

LTA-POC is a rapid test for the measurement of Lymphotoxin-α
(LTA) in
human
tears. It is intended for monitoring ocular surface immune
homeostasis
and
facilitating clinical diagnosis of dry eye disease. LTA is a
valuable
biomarker for the assessment of immune homeostasis, which is an
important
factor in dry eye pathogenesis.
The lack of correlation between clinical signs and symptoms of
dry eye
makes
quick and accurate diagnosis difficult. Currently, Schirmer's
test, tear
break-up time, keratoconjunctival staining, tear osmolarity are
the
commonly
used clinical tests for dry eye diagnosis. However, without
objective,
quantitative diagnostic tests that correlate well with and the
severity
of
dry eye, the objective differentiation of dry eye symptoms from
other
eye
diseases that present similar symptoms, such as ocular
allergies,
conjunctivochalasis or infectious bacterial or viral diseases,
poses a
significant challenge to timely diagnosis and treatment.
Biomarker profiling with tear fluid indicates that immune
homeostasis is
lost in the ocular surface in dry eye disease, with patients
suffering
from
dry eye have significantly lower LTA levels in their tears as
compared
with
control groups1.
····························································································································································································································
LTA and Immune Homeostasis
Regulation of ocular immune tolerance is important for the
homeostasis
and
protection of the ocular tissues from pathogen invasion and
immune-medicated
inflammation and immune-mediated injury. Immune homeostasis is
critical
for
the protection of ocular surface health, and the loss of immune
homeostasis
can lead to immunopathology1. Regulatory T cells
(Tregs) are
suppressive
T
cells that play an essential role in maintaining the balance
between
immune
activation and tolerance2. The quantity and activity
of Tregs
are
critical
in maintaining the immune homeostasis of ocular
surface3.
Tumor necrosis
factor receptor 2 (TNFR2) is expressed highly and selectively in
Treg,
and
the activation of TNFR2 is essential for the activity and
function of
Tregs4. Lymphotoxin-α (LTA) is a ligand for TNFR2.
The
binding of LTA to
TNFR2 activates the proliferation
and
function of Tregs; therefore, the
levels of LTA reflect the status of immune homeostasis in the
ocular
surface.
····························································································································································································································
LTA-POC measures the levels of LTA to monitor ocular immune
homeostasis
and
facilitate rapid, accurate diagnosis of dry eye.
····························································································································································································································
References
1.
Huang JF, Lin X, Liu ZG. Altered Lymphotoxin alpha (LTA)
level in
tear
fluid, measured with a POCT test, in dry eye patients.
Invest.
Ophthalmol.
Vis. Sci. 2018. 59(9):955
2.
Sakaguchi S1, Yamaguchi T, Nomura T, Ono M. Regulatory T
cells and
immune
tolerance. Cell. 2008;133(5):775-87.
3.
Foulsham W1, Marmalidou A1, Amouzegar A1, Coco G1, Chen Y1,
Dana R2.
Review: The function of regulatory T cells at the ocular
surface. Ocul
Surf.
2017;15(4):652-659.
4.
Wang J, Ferreira R, Lu W, Farrow S, Downes K, Jermutus L,
Minter R,
Al-Lamki RS, Pober JS, Bradley JR. TNFR2 ligation in human T
regulatory
cells enhances IL2-induced cell proliferation through the
non-canonical
NF-κB pathway. Sci Rep. 2018. 8(1):12079
IgE POC is a rapid test for the measurement of Immunoglobulin E
(IgE) in
human tears. It is intended to facilitate clinical diagnosis of
allergic
conjunctivitis. IgE is a valuable biomarker for diagnosing
allergic
conjunctivitis related to the type I hypersensitivity response.
Currently, the diagnosis of allergic conjunctivitis is usually
based on
clinical history, symptoms and signs. However, because of the
overlapping
symptoms between allergic conjunctivitis and other eye diseases
such as
dry
eye, objective, quantitative diagnostic testing for allergic
conjunctivitis
is greatly needed to avoid inaccurate diagnosis and treatments.
IgE concentration in human tears is normally very low in the
absence of
disease. Elevated total IgE levels in the tears are seen in
patients
with
allergic conjunctivitis.
····························································································································································································································
IgE and Allergic Conjunctivitis
IgE mediates type I hypersensitivity response and plays a key
role in
the
pathogenesis of allergic conjunctivitis. Type I hypersensitivity
is
characterized by the occurrence of allergic reactions
immediately
following
re-exposure to an allergen. The ocular hypersensitivity response
results
from exposure of the conjunctiva to environmental allergens that
stimulate
the IgE production. Binding of allergens and cross-linking of
IgE on the
surface of sensitized mast cells and basophils results in cell
degranulation, leading to the release of inflammatory mediators
and the
development of allergic diseases.
····························································································································································································································
IgE POC measures the total IgE levels to facilitate rapid,
accurate
diagnosis of type I allergic conjunctivitis.
MMP-9 POC is a rapid test for the measurement of Matrix
Metallopeptidase
9
(MMP-9) in human tears. It is intended to monitor inflammation
of the
ocular
surface and facilitate diagnosis of dry eye disease. MMP-9 is a
valuable
biomarker for monitoring ocular surface inflammation, a core
underlying
mechanism in chronic dry eye, and for stratification of dry eye
subtypes.
Currently, Schirmer's test, tear break-up time,
keratoconjunctival
staining,
tear osmolarity are the commonly used clinical tests for dry eye
diagnosis;
however, these tests cannot identify the exact pathological
mechanisms
of
the kind of dry eye subtype. Understanding the pathogenesis and
pathological
processes of dry eye is critical in clinical practice.
The MMP-9 activity in tears and MMP-9 gene expression were
reported as
relatively low in normal subjects and elevated in the patients
with dry
eye.
····························································································································································································································
MMP-9 and Inflammation
MMP-9, an inflammatory marker, is a zinc-binding proteolytic
enzyme
produced
by stressed basal corneal epithelial cells and neutrophils.
Proteolytic
activities of MMPs play an important role in vascular
remodeling,
cellular
migration, and the processing of ECM proteins and adhesion
molecules.
MMP-9
consists of a prodomain, catalytic domain, hinge region, and a
hemopexin
domain, and is secreted as an inactive zymogen that becomes
activated
extracellularly. The most relevant natural activators of
proMMP-9 are
unknown. MMP-9 activation may be mediated by removal of the
prodomain by
serine proteases or other MMPs, or it may be a direct response
to
oxidative
stress that disrupts the cysteine switch. MMP-9 is capable of
processing
cytokines and chemokines.
····························································································································································································································
MMP-9 POC measures the levels of MMP-9 to assess ocular
inflammation and
facilitate rapid, accurate diagnosis of dry eye, as well as the
stratification of dry eye subtypes.
i-ImmunDx™ Analyzer
The i-ImmunDx™ Analyzer is a highly sensitive reader, designed to read
Seinda's POC
test cassettes.
Fast,
easy-to-use use interface that allows operators to test
patient samples and navigate stored results with speed
patient samples and navigate stored results with speed
Small,
light-weight, and portable, suitable for in outpatient,
clinical inspection, laboratory, and other POC settings
clinical inspection, laboratory, and other POC settings
Two test
modes, allowing the operator greater flexibility to
accommodate to workflow demands
accommodate to workflow demands
Automated
results that support qualitative,semi-quantitative,
and quantitativein vitro immunoassays
and quantitativein vitro immunoassays

To Order
To purchase or request more information, send us an email at sales@seindabio.com
or send us a message.